We’re pursuing innovative science with antibody-drug conjugate (ADC) research
Merck scientists are evaluating ADCs to explore novel treatment approaches in both solid tumors and blood cancers
May 19, 2025
3D depiction of an antibody-drug conjugate molecule
What are antibody-drug conjugates (ADCs)?
ADCs are a targeted means to transport and deliver chemotherapy to tumor cells. More than two decades since the first approval of an ADC, scientists continue to explore how, by leveraging novel scientific advancements, they can find new ways to better design and develop these molecules in order to better address current unmet needs in cancer treatment.
ADCs are made up of three distinct yet equally important elements — an antibody, a linker and a cytotoxic drug/chemotherapy payload. These elements work together to transport the chemotherapy payload to a specific target expressed on the surface of a cancer cell, bind to the target and then be absorbed into the cell to release the chemotherapy.
Anatomy of an ADC
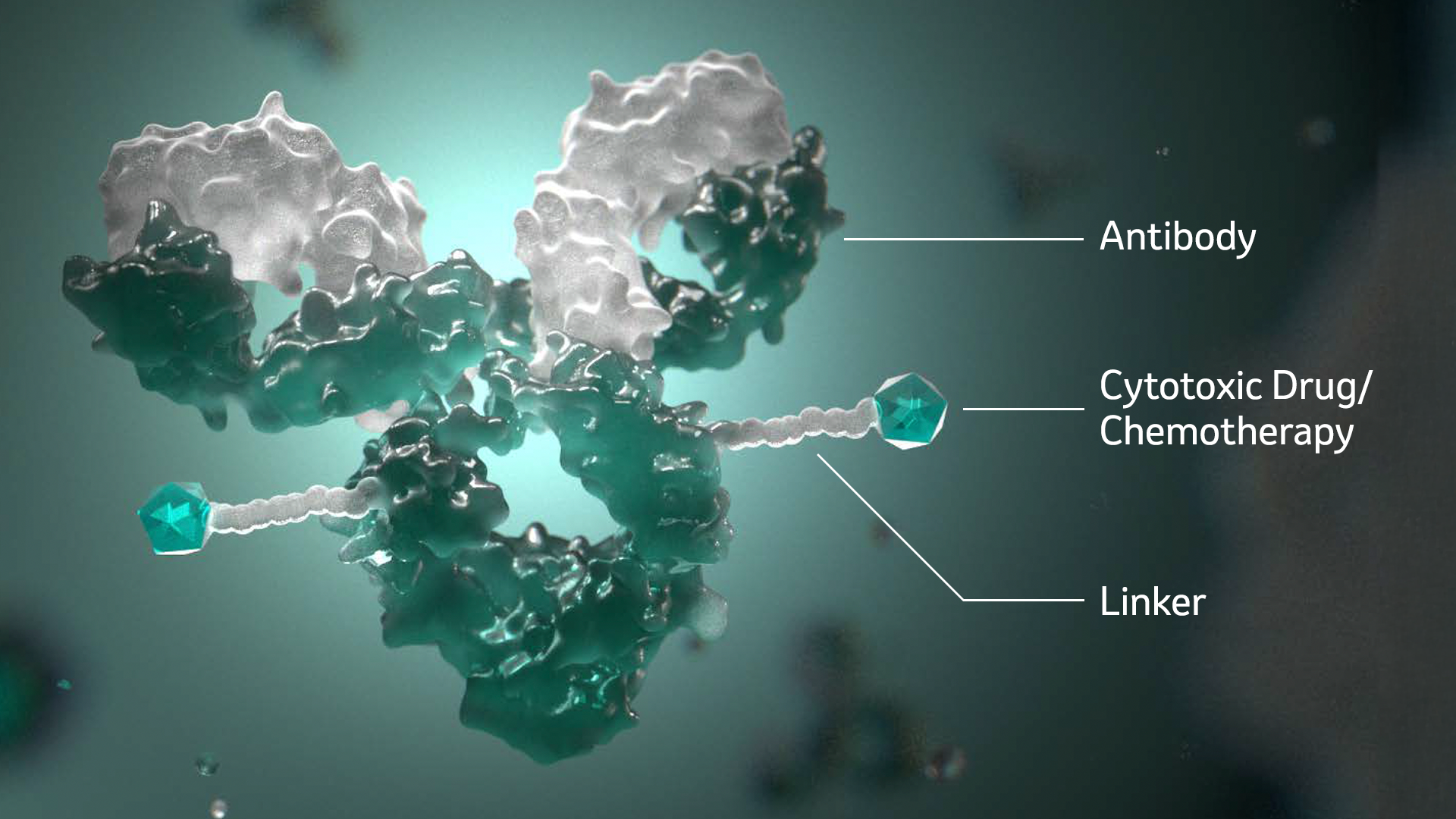
- The antibody serves as the targeting mechanism, like a zip code helping to direct the delivery of the chemotherapy agent to cancerous cells.
- The payload, or the chemotherapy agent, is responsible for working to destroy the cancer cell when it’s released.
- The linker attaches the chemotherapy agent to the antibody and triggers the release of the chemotherapy agent once inside a cancerous cell.
Watch an animation of an ADC in action
Advancements in the scientific research behind ADCs
Since ADCs were first introduced in 2000, the chemistry and science behind these molecules has advanced significantly, and scientists have developed a greater understanding of what makes a good ADC target and how to design ADCs more effectively.
Tumor antigens, or proteins expressed on the outside of a cancer cell, are what an antibody initially binds to. Scientists now know that characteristics like how quickly an antigen is brought inside the cell and whether an antigen is recycled back to the cell surface are crucially important.

“Imagine ADCs as specialized agents that recognize and bind to specific tumor antigens on cancer cells. Once they attach to these antigens, ADCs are internalized by the cell, allowing the chemotherapy agent to be released directly inside, delivering the treatment where it’s needed most — at the core of the cancer cell.”
- Dr. Omobolaji Akala
Associate vice president of oncology early development, Merck Research Laboratories
There have also been notable advancements in both linker and payload chemistry. Scientists have been focused on improving the stability of linkers and reducing the risk of payload release in the body, as well as evaluating where and how many molecules can be incorporated into the payload. Combined, these advancements may help in reducing damage to nearby healthy cells, while releasing a potent payload precisely within a cancer cell.
This evolving understanding of the science is fueling innovative research efforts with the goal of bringing more effective ADCs to patients.

“We’ve learned that designing ADCs is about balancing the right level of tumor antigen expression, the right potency of a cytotoxic agent in the payload, addressing the cell biology through that payload and engineering a payload to release at the right time and in the right place.”
- Dr. Marjorie Green
Senior vice president and head of oncology, global clinical development, Merck Research Laboratories
Exploring ADC targets
We’re focusing on proteins associated with poor prognosis across both solid tumors and blood cancers to expand the impact of ADC therapies and address the needs of more patients. Our scientists are also combining ADCs with other innovative treatments such as immunotherapies and T-cell engagers.
By exploring the potential of a broad range of ADC targets and applying new technologies — such as novel linker chemistries, optimized payloads and combination strategies with other therapies — we aim to deepen our understanding of these complex molecules and work to identify and develop new meaningful therapeutic options for patients, aligning with our purpose of using the power of cutting-edge science to save and improve lives around the world.
Learn more about our work in oncology.



