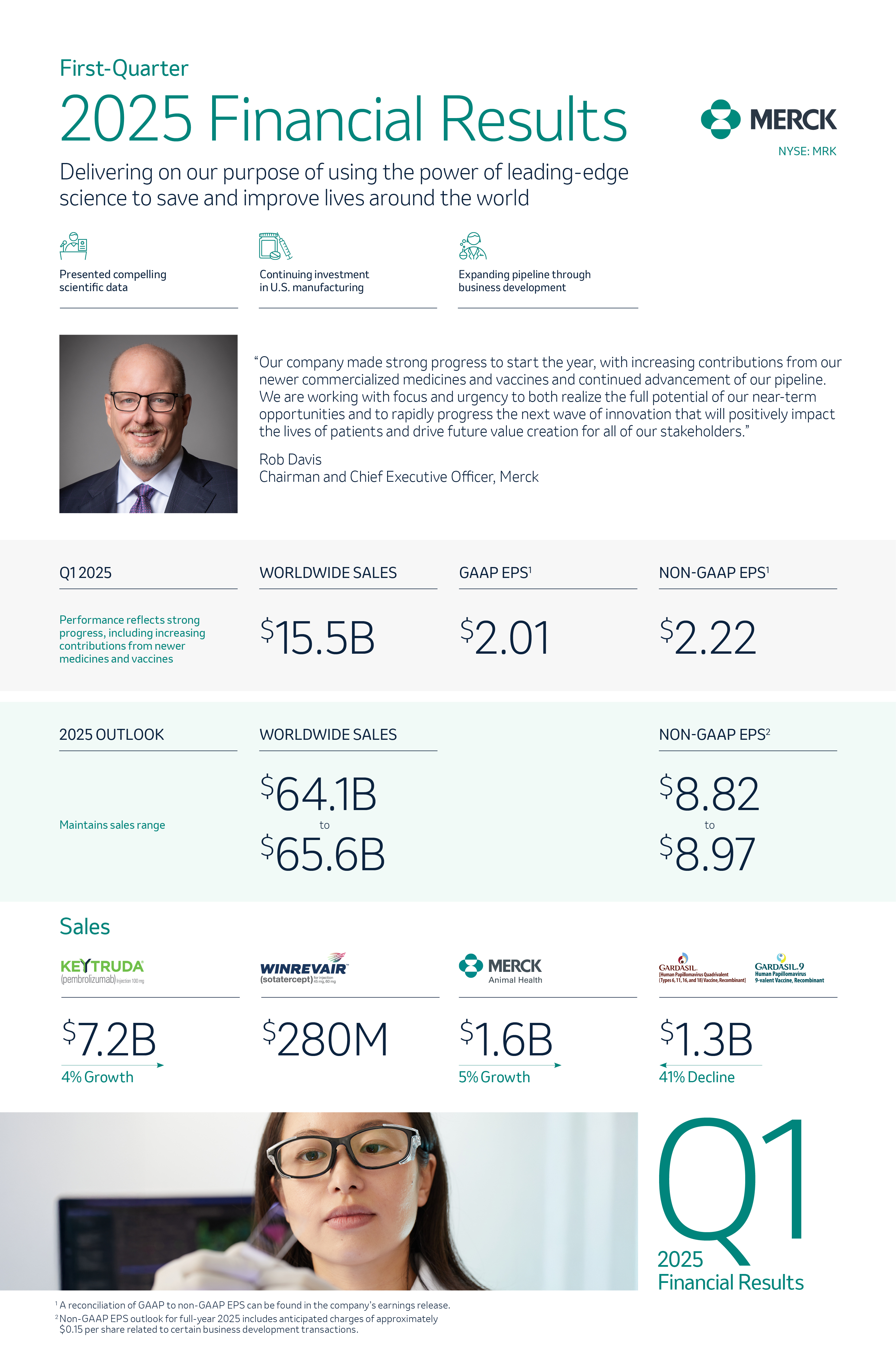
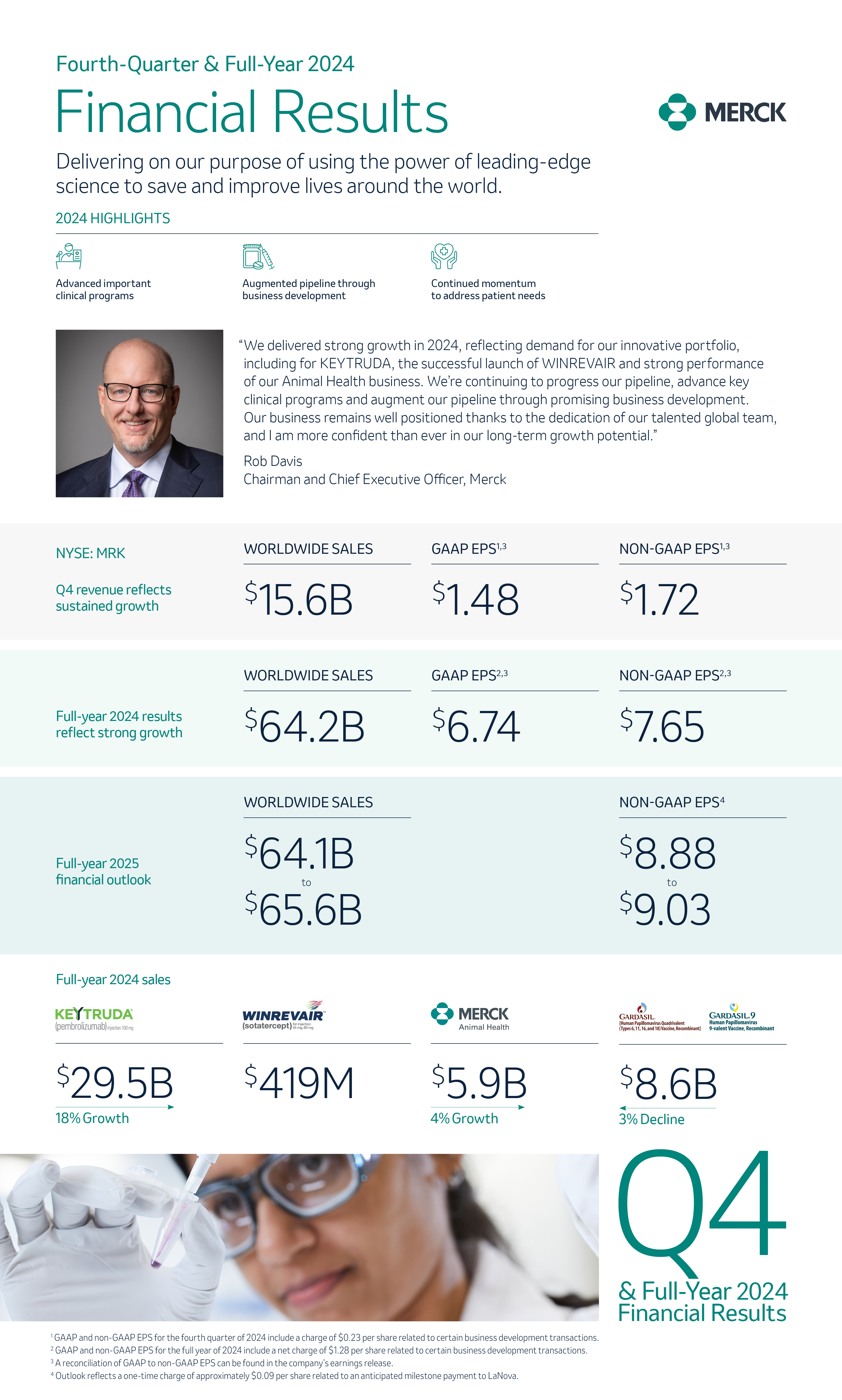
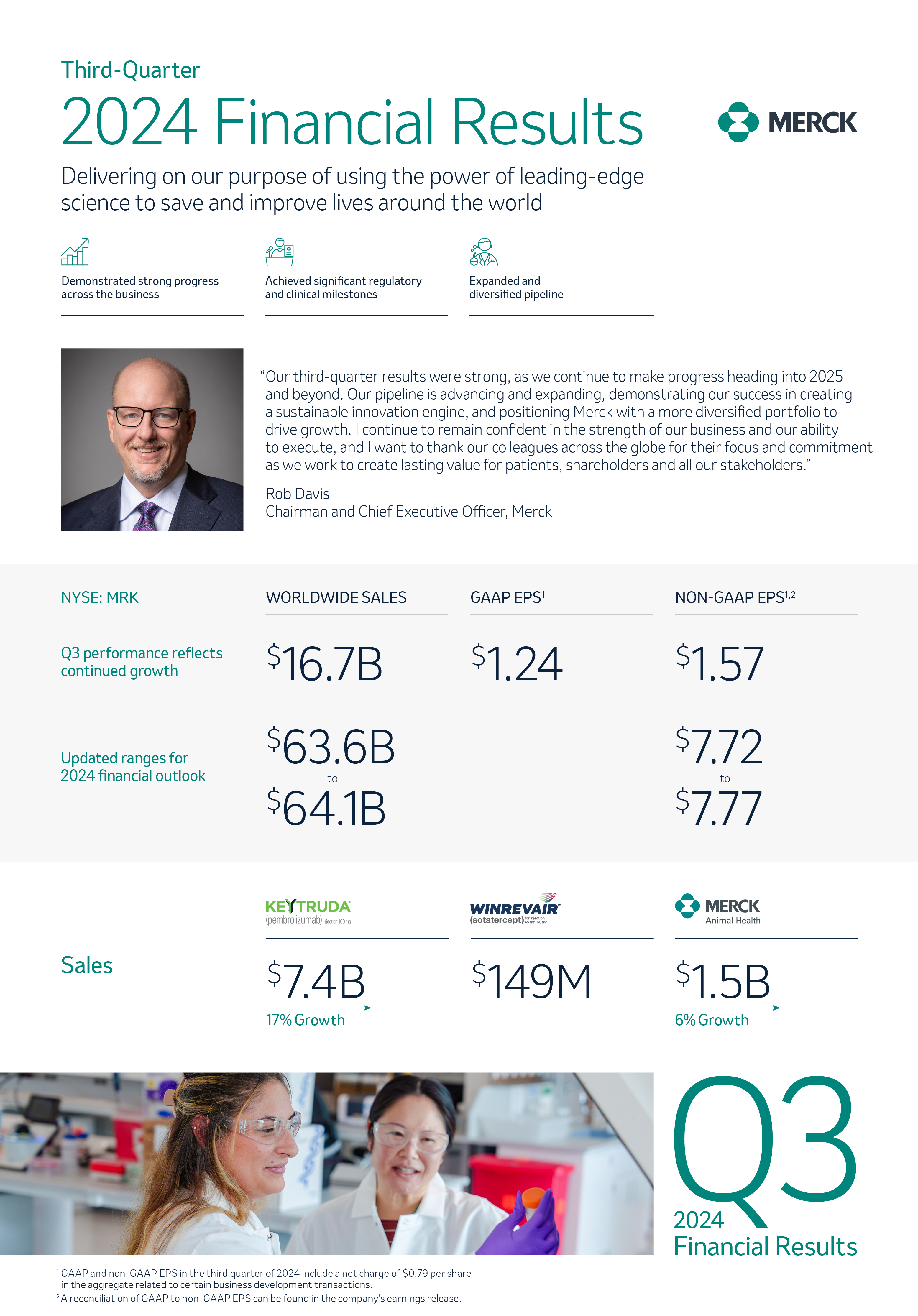
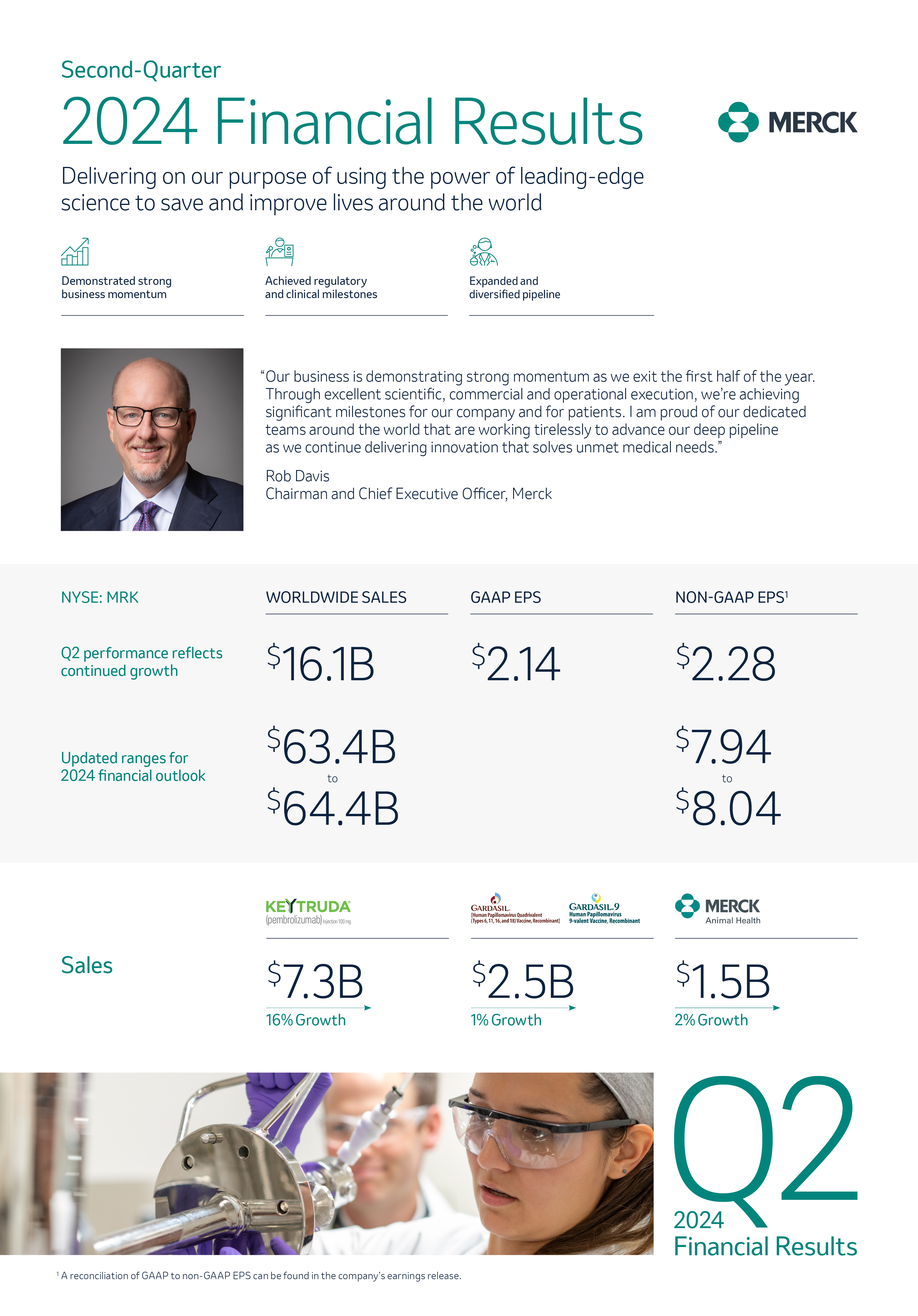
Innovation
TL1A: Exploring a potentially important target for inflammatory bowel disease
Our scientists are investigating ways to modulate TL1A to potentially address inflammation and fibrosis associated with inflammatory bowel disease
Picture the immune system as a carefully orchestrated network of organs, cells and molecules working together to protect your body from foreign invaders. Normally, this system operates smoothly, identifying and then fighting off microbes. However, in the case of inflammatory bowel disease (IBD), a person’s immune system can mistakenly attack their own tissues, causing chronic inflammation and tissue damage.
Nearly three million people in the U.S. live with Crohn’s disease or ulcerative colitis, the two most common forms of IBD. Patients with IBD experience symptoms such as diarrhea, blood in the stool, abdominal pain, unintended weight loss, fatigue and impaired sleep. In 25-40% of patients, IBD may affect organs and tissues outside of the gastrointestinal system, including the joints, skin, bones, eyes, kidneys and liver. When IBD is uncontrolled, it can lead to hospitalizations and surgery.
Despite available therapies which have helped improve patients’ symptoms over the last two decades, many patients do not achieve a state of sustained remission.
“People with IBD often struggle to find a treatment that works for them because each individual’s disease is different,” said Aileen Pangan, vice president and therapeutic area head, immunology clinical research, Merck Research Laboratories.
“With a better understanding of the biology of IBD, medicines have begun to emerge that aim to change the way we approach treatment. We’re investigating ways to modulate targets, including TL1A, that have been implicated in IBD and other immune-mediated inflammatory diseases.”
Our TL1A research
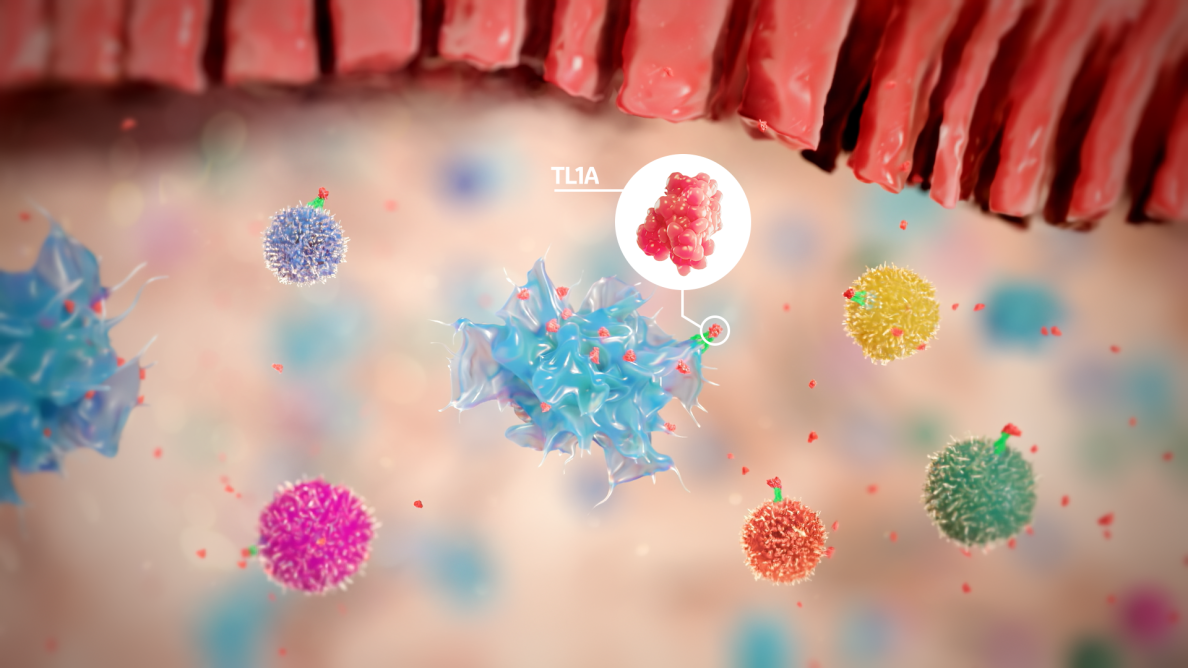
Our scientists are investigating tumor necrosis factor-like ligand 1A (TL1A), which has been shown to be increased in inflamed intestinal tissue and in the systemic circulation of patients with IBD.
TL1A is a cytokine, a protein functioning as a chemical messenger, that acts as a regulator of cellular immunity. In healthy people, levels of TL1A increase to help immune cells fight infections effectively, with levels going back down after the infection is gone. However, in IBD, TL1A levels are chronically elevated, leading to an excessive buildup of immune cells in the digestive tract, chronic inflammation, tissue damage and fibrosis.
Teams at Merck are evaluating the potential role of TL1A across immune-mediated inflammatory diseases, including Crohn’s disease, ulcerative colitis and systemic sclerosis-associated interstitial lung disease (SSc-ILD). Learn more about our research in immunology.