
Our therapeutic modalities
We’re harnessing the power of cutting-edge science and a breadth of state-of-the art scientific technologies to create future medicines and vaccines.
Emerging modalities enable our pipeline
Improvements in our understanding of disease biology and a growing arsenal of therapeutic modalities are fueling progress in drug discovery. Traditionally, modalities — or the type of molecule used to prevent or treat a disease — have been limited to small (predominantly oral) and large (predominantly injectable) molecules. But as we set out to identify new ways to reach new biological targets and treat and prevent disease, we’ve diversified our scientific approach to include emerging modalities such as macrocyclic peptides, multi-specific biologics, targeted drug conjugates and new vaccine technologies.
Our expanded toolbox of therapeutics
Our scientists strategically select these modalities with the goal of tackling complex disease pathways that could lead to improved outcomes for patients. Our expanded toolbox helps us advance the most promising opportunities across our core areas of cardiovascular disease, immunology, infectious diseases, neuroscience, oncology, ophthalmology and vaccines.
Small molecules
Small molecules are lower molecular weight organic compounds (100-1000 Da). Because of their small size, they can more easily diffuse across cell membranes and can generally be taken as a pill. They’ve been a mainstay in the treatment for a variety of diseases and make up approximately 90% of medicines used today. We’re designing small molecules to engage a variety of tissue and cellular targets involved in a wide range of diseases. Our development efforts include newer classes of small molecules, covalent inhibitors or targeted protein degraders.

Macrocylic peptides
Macrocyclic peptides are engineered peptides that are structurally modified with the potential to more selectively bind to a drug target, and the goal of mimicking a biologic in their functionality. They’re intended to combine the potential for oral administration that comes with small molecules with the potency and target specificity of an antibody.
Monoclonal antibodies
Monoclonal antibodies (mAbs) are discovered and designed to bind to specific molecular targets to inhibit or modulate a biologic activity with the goal of helping improve disease outcomes. Therapeutic mAbs often target pathogens circulating in the blood or molecules on the surface of cells, including receptors and antigens. We’re also investigating the use of mAbs to diversify the prevention landscape by providing rapid protection against certain infectious diseases through passive immunity.
Multi-specific antibodies

Multi-specific antibodies are engineered on an antibody framework to have multiple distinct binding domains that can bind to various targets, with the goal of providing improved benefit over binding a single target.
T-cell engagers are a type of bispecific antibody designed to recruit and activate T cells to recognize and attack tumor cells by triggering the release of cytotoxic molecules that work to kill the tumor.
Fusion proteins
Fusion proteins link multiple protein domains into longer functional proteins. Often functional domains of natural human proteins are fused to antibody frameworks designed to create a biotherapeutic with a certain biological function and duration of action.
Antibody-drug conjugates (ADCs)
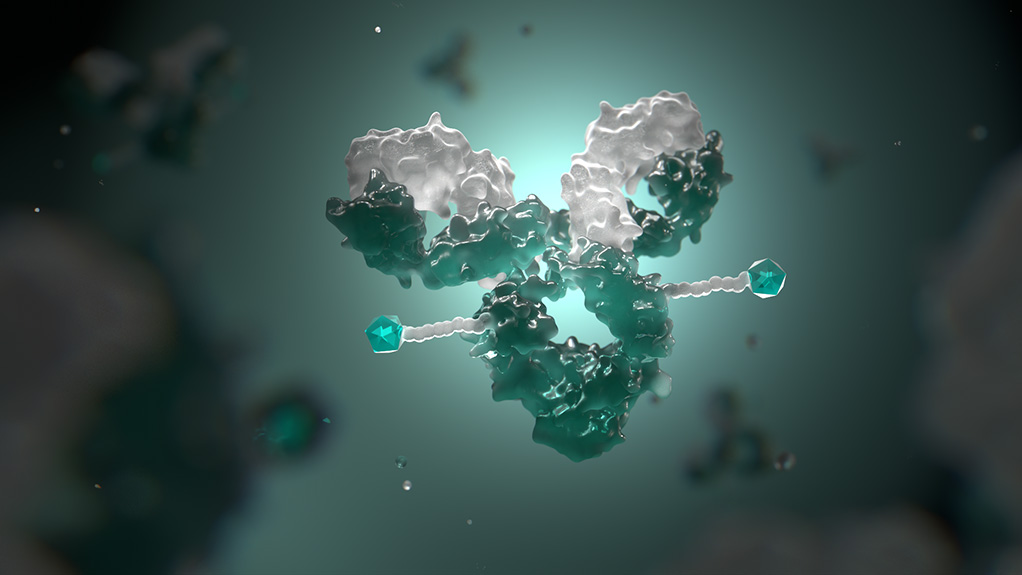
Antibody-drug conjugates (ADCs) are designed to provide a targeted means of delivering medicine where it’s needed. In oncology, ADCs consist of an antibody, a linker and a payload of cytotoxic (cell-killing) agent, such as chemotherapy. The ADC, through the antibody, binds to a specific target — a protein or receptor — expressed on the surface of a cancer cell. The ADC is then absorbed into the cell where the chemotherapy is released and works to kill the cell.
Peptide-drug conjugates (PDCs)

Peptide-drug conjugates (PDCs) are considered the next generation of targeted therapeutics after ADCs. PDCs use a carrier peptide conjugated to a small molecule cytotoxin, such as chemotherapy, to precisely bind to the target and deliver the payload, which is the cytotoxin that kills the cancer cells. PDCs have distinct features as carriers, which may include low immunogenicity, differentiated biodistribution and less complex manufacturing.
RNA technology
We’re building on recent innovations with messenger ribonucleic acid (mRNA) by researching new RNA-based approaches that can be applied to preventative vaccines and highly personalized cancer therapies. Individualized neoantigen therapies harness mRNA technology to precisely target a patient’s unique cancer cells with an antitumor immune response.

Vaccines
Vaccines are biological preparations designed to teach the immune system to recognize and defend against disease-causing microorganisms, such as viruses and bacteria. Different vaccine technologies can be used to generate a vaccine depending on the pathogen. We’re diversifying across viral, protein and RNA-based vaccines. We’re also investigating the use of monoclonal antibodies to diversify the prevention landscape by providing rapid protection against certain infectious diseases through passive immunity.
Live attenuated virus vaccines
Some of the most common childhood vaccines are live attenuated virus vaccines. These vaccines use a weakened form of a virus that do not cause disease but can still replicate just enough to train the immune system. This close imitation of natural infection typically elicits broad, long-lasting immunity and has formed the basis of many long‑standing preventive vaccine programs.
Protein subunit vaccines
Protein‑based vaccines contain purified pieces of a pathogen — either a single protein (subunit) or a protein chemically linked to a carrier molecule (conjugate) — that safely mimic the pathogen and prompt a protective immune response.
“We know more about diseases than ever before. With this depth of disease biology expertise, we recognize that we cannot limit ourselves to any single technology in drug development. Our modality-enabled approach means we aim to choose the right tools — from emerging modalities to proven scientific approaches — to design medicines and vaccines with the potential to address unmet needs across a wide range of diseases.”
- Dr. Robert Garbaccio
Head of discovery chemistry, Merck Research Laboratories

R&D careers at Merck
We’re exploring a diverse toolbox of modalities to develop new medicines and vaccines with a goal of helping to save and improve lives — now and in the future. Join our team of innovators today.









