How we’re addressing health literacy
Many people struggle to understand health information, which can impact health outcomes. What we're doing to support health literacy.
December 6, 2024

What is health literacy?
At points in our lives, we need to make health or medical decisions for ourselves, members of our family or those in our care. And sometimes it can be challenging. Whether it’s deciding to receive a vaccine, start a new medication, undergo a procedure or join a clinical trial, our level of health literacy — the ability to find, understand and use information and services — can play an important role in health outcomes. Unfortunately, when people struggle, there may be negative health consequences, like decreased adherence to treatment plans, increased emergency room visits and hospital stays, and higher mortality rates.
Low health literacy is more common in vulnerable populations
While limited health literacy can affect anyone, there are certain populations at greater risk: older adults, racial and ethnic minorities, those with low income or less education, and people with compromised health status.
“As a company committed to helping save and improve lives, it’s our responsibility to communicate in a way that people can understand so that they can make informed health decisions,” said Shehla Hussain, director, medical writing safety services.
Health literacy is important for health equity
People with better health literacy are more likely to proactively engage in disease preventive behaviors and make informed decisions about their well-being. On the other hand, people with lower or limited health literacy may struggle to understand relevant information, leading to an increased risk of poor health outcomes.
How we’re making medical information easier to understand
We’re committed to making sure the information we share with the world is very clear. It’s something we’ve focused on since 2011, long before the U.S. Department of Health and Human Services updated the definition of health literacy in 2020 to acknowledge that organizations have a responsibility to equitably enable individuals to find, understand, and use information and services to inform health-related decisions and actions for themselves and others.
As part of our efforts, we created a plain language glossary and established company-wide standards focused on improving the health literacy of the information in our resources and materials. It starts in product development and continues through the life cycle of the product, including clinical trials, labeling, post-approval, marketing and promotional materials. For example, we’re:
- Creating easy-to-read patient labels.
- Improving packaging and instructions for use.
- Developing easy-to-understand disease educational materials.
- Improving health literacy within clinical trials.
- Expanding our plain language glossary of medical/scientific terms
- Supporting research on health literacy.
- Utilizing plain language and graphics in digital and online resources.
- Sharing best practices externally.
We also listen to the people who use or may use our products to help guide our efforts.

“We work to build trust by listening to the communities we serve, understanding their needs and making our information clear, concise and understandable.”
– English D. Willis
Executive director, clinical safety and risk management, and executive sponsor, Health Literacy Community of PracticeReducing health disparities around clinical trials
Within our clinical trials, we strive to reduce health care disparities by increasing diverse representation in our clinical trials.
“Health literacy is critically important for achieving clinical trial diversity as it ensures that individuals from diverse backgrounds can understand trial information, make informed decisions and effectively participate. Improving health literacy is essential for equitable access and participation, fostering greater inclusivity in research, leading to more representative and impactful outcomes” said Luther Clark, executive director, medical affairs, patient innovation and engagement.
Euvon Jones, a clinical trial participant, said: “Knowledge is power, and during my journey with prostate cancer, I realized the importance of fully understanding my diagnosis and the options available to me. Through conversations with my doctor and loved ones, and seeking information from reliable sources, I felt empowered to make informed decisions. When we fully understand the importance of the health information we receive, we’re better equipped to navigate the health care system, communicate with providers and advocate for the best possible care.”
Look at some examples of our work

Defining complex medical terms in plain language
By defining common terms used by health care providers, patients feel more prepared and confident to engage in discussions with their care teams.
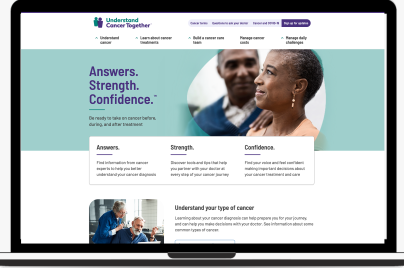
Easy to navigate website
Understanding the patient's perspective allowed us to create a website that anticipates their needs for easy navigation. Plainer language, effective visuals and simple layouts make the website a useful tool.

Seeing from the patient perspective
In a diabetes awareness brochure, we presented the view from the patient's perspective so they can see the potential effects of eye damage from diabetes.
“And our work isn’t done. While we continue to learn and engage with communities, we’re also learning better ways to communicate with our consumers, patients and the general public.”
– English D. Willis