A lifelong commitment to addressing dengue fever
How our colleague’s personal experience fueled his desire to help others
June 10, 2025
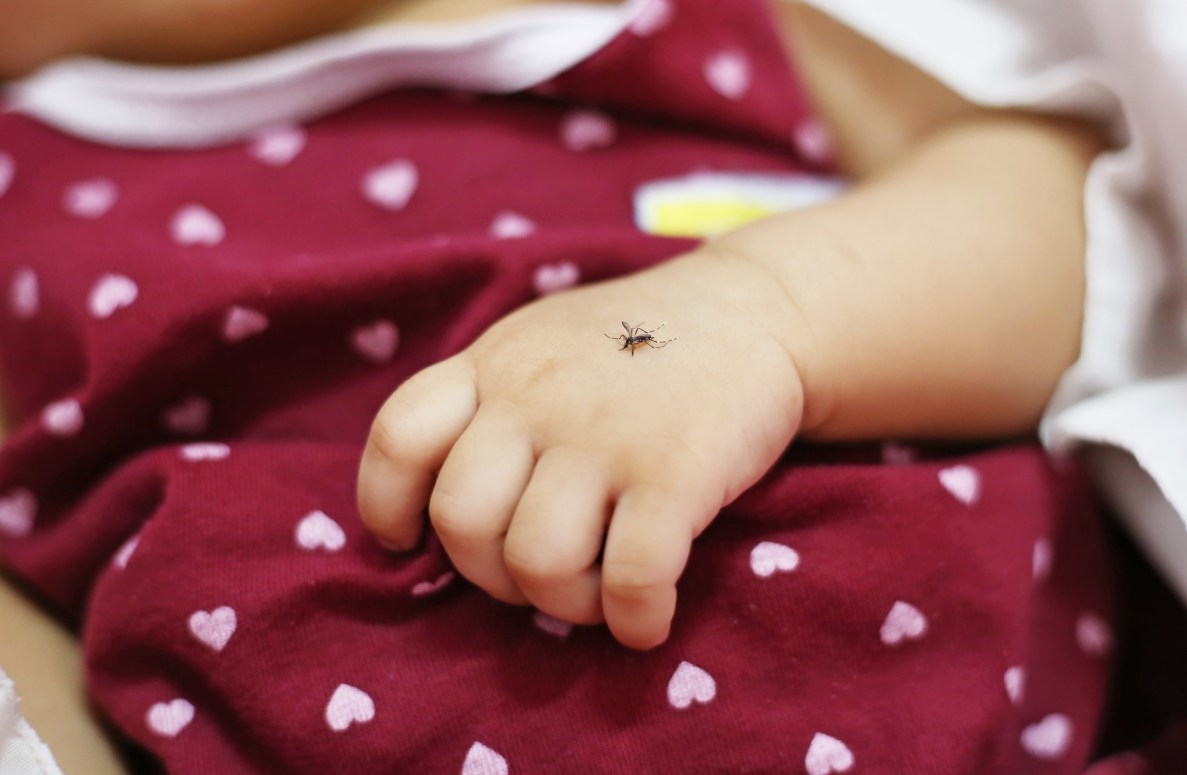
With roughly half of the world’s population at risk of contracting dengue fever, Dr. Bin Pangilinan and his colleagues are dedicated to helping address this global health challenge.
Growing up in the Philippines, Pangilinan experienced firsthand the serious impact of dengue, one of the fastest-growing mosquito-borne viral diseases. He saw classmates become ill from the disease and, even though it was not contagious, unfortunately contracted it himself from infected mosquitos multiple times throughout his childhood. The care he received helped shape the course of his life in profound ways.
Pangilinan around 7 years old
His first encounter with dengue at age 7 landed him in the hospital, an experience marked by high fevers and severe body aches. “You would hear about outbreaks in the news, but being at the center of one was entirely different,” he said.
At age 10, a more severe episode required a two-week stay in the hospital — alarming both him and his parents. Finally, at age 15, Pangilinan was diagnosed with dengue once again, a third hospitalization disrupting his college admission tests and highlighting the disease’s unpredictability.
Joining the fight against dengue
His experience with dengue as a child ignited Pangilinan’s passion for medicine, driving him to pursue a career helping others. After completing his medical training, he faced dengue from the other side as a medical intern. “It was one of the most challenging moments in my medical career,” he said. “Treating patients impacted by dengue was a pivotal moment that further fueled my desire to help address this disease.”

Pangilinan during his medical training
Pangilinan’s career journey eventually led him to Merck in 2013, where he’s worked with various groups including advocacy, medical affairs, access and policy to help address infectious diseases. His personal journey inspires his professional work with a unique empathy and understanding, particularly in his current role, where he is part of a team supporting efforts to alleviate the burden of dengue and infectious diseases.
“The commitment of my colleagues and the dedication of the global public health community to fight dengue are incredibly inspiring,” Pangilinan said.
“That passion energizes me every day and drives me to confront this disease and make a difference for those at risk around the world, especially for my hometown in the Philippines, where my story with dengue began.”
Pangilinan and his colleagues remain committed to leveraging science, innovation and collaboration to help address the impact of infectious diseases like dengue on communities around the world.